Persistence Market Research (PMR) has recently published a report titled “Host Cell Contaminant Testing Market: Global Industry Analysis (2012 – 2016) and Forecast (2017 – 2025).” The report states that the healthcare industry is transforming at a rapid pace and is venturing into different possibilities for research and development of new technologies.
Several government and regulatory bodies of various countries are adopting various cost containment measures to reduce healthcare burden, especially in developed economies. This shift from volume- to value based system is driven by various measures taken by governments, providers, payers and life sciences companies, which include outcome-based pricing, profit and risk sharing, price control and competitive tendering.
Get Sample Copy of this Report@ https://www.persistencemarketresearch.com/samples/21355
The value-based healthcare system will help in achieving maximum value for the money spent on healthcare and thus, improve the outcomes through integrated care pathways, but at the same time will levy pricing pressure on the biopharmaceutical companies and may impact the investment in R&D of innovative medical treatment technologies.
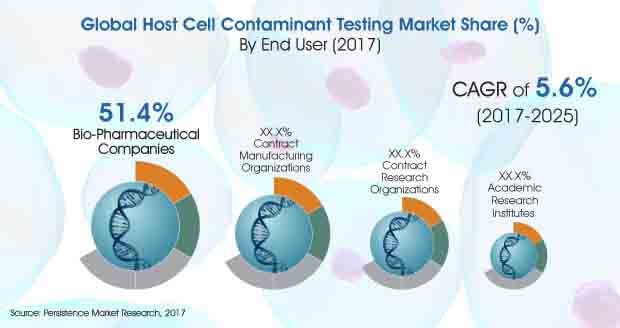
The global market for host cell contaminant testing market is expected to rise at a stable CAGR of 5.6% from 2017 to 2025. The market is expected to touch a US$ 354 Mn by the end of 2025.
- BioGenes GmbH
- Bio-Rad Laboratories, Inc.
- Cisbio Bioassays
- Cygnus Technologies LLC (A Maravai Lifesciences Company)
- Enzo Life Sciences, Inc.
- ForteBio (A Pall Company)
- GE Healthcare Life Sciences
- Molecular Devices LLC
- ProteinSimple (A BioTechne Brand)
- Thermo Fisher Scientific, Inc.
- Others
Request for Methodology@ https://www.persistencemarketresearch.com/methodology/21355
Bio-Safety of Drugs to be Key Growth Driving Factor
Biopharmaceutical products manufactured from biological systems may be contaminated with host cell components. Host cell contaminants constitute major parts of process-related impurities and can severely affect drug safety. Even though low levels of most of the host cell contaminants may be insignificant, but due to patient safety host cell contaminants are reduced to low levels or eliminated to reduce adverse immune reactions.
Failure to identify host cell contaminants in early stages of manufacturing results in reduced drug efficacy or adverse reactions on patients. Biosafety of patient population is primary requisite, host cell contaminant testing is performed at each and every step of biopharmaceutical manufacturing to eliminate the risk of host cell contaminants.
However, the diversity of proteins which is within the host cell contaminants is expected to pose various challenges in the testing of contaminants in the coming years. Dearth of coverage for non-immune reactive host cell contaminants and lack of identification and specificity are expected further hinder the growth of the market in the long run.
Access Full Report@ https://www.persistencemarketresearch.com/checkout/21355
North America Topping the Charts with High Adoption Rate
Region wise, the host cell contaminant testing market is sectioned into five key regions namely Latin America, North America, Asia Pacific (APAC), Europe, and the Middle East and Africa (MEA). The North America has turned into the lucrative market for host cell contaminant testing and is expected to rise as the quickest developing business sector because of activities like estimation of biotechnology programs that help the United Kingdom’s National Measurement System.
Asia Pacific area is relied upon to enlist a huge development over a figure period. Europe is additionally anticipated to enlist a noteworthy development in the host cell contaminant testing market over a conjecture period.
Based on product type, ELISA based assays segment is expected to occupy a huge share in the global cell contaminant testing market. On the basis of application, the market was led by product manufacturing.
